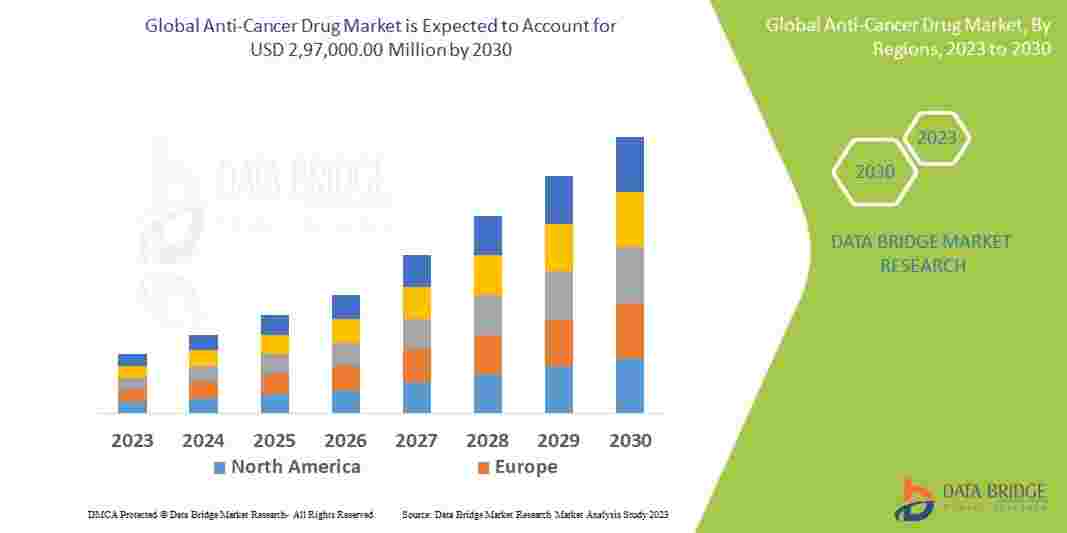
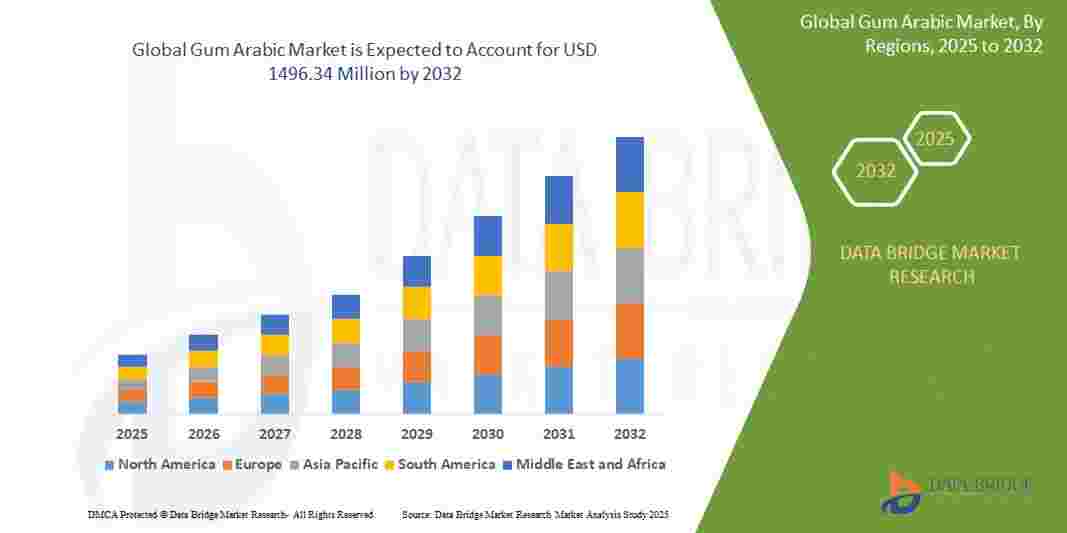
Regulatory Tightrope: The Urgent Need for Robust Tracking Systems to Maintain Global Pharmaceutical Compliance
The pharmaceutical industry operates under a dense and constantly shifting web of global regulations, covering everything from clinical trial conduct (Good Clinical Practice, GCP) to manufacturing quality (Good Manufacturing Practice, GMP) and post-market safety reporting. Maintaining compliance across multiple jurisdictions is a gargantuan task, with failures leading to massive fines, product seizures, and significant damage to corporate reputation. As globalization continues, the need for robust, analytical tracking systems that can monitor regulatory changes in real-time across the world has become a non-negotiable requirement for operational success.
Commercial analytics and intelligence systems are now being adapted to provide comprehensive regulatory compliance tracking. These systems use natural language processing to monitor regulatory agency announcements, proposed rule changes, and enforcement actions from bodies like the European Medicines Agency (EMA) and the Japanese Pharmaceuticals and Medical Devices Agency (PMDA). This proactive intelligence allows companies to update internal protocols, manufacturing processes, and product labeling before new regulations are enforced, minimizing risk. The complexity of ensuring consistency across various international markets—each with unique labeling and data submission requirements—is a major driver for investment in this technology.
A specific area of high growth involves tracking and managing compliance for global drug safety and pharmacovigilance reporting, which requires timely and accurate submission of adverse event data. Failure to report promptly can result in significant penalties. Sophisticated analytics systems automate the collection and standardization of this data across different territories. Companies are increasingly seeking technology solutions for this challenge, boosting the market for commercial analytics focused on Regulatory compliance tracking systems and risk management.
The financial incentive to invest in these systems is clear: regulatory non-compliance fines can reach into the hundreds of millions, far outweighing the cost of the analytical infrastructure. Given the estimated $10 billion spent annually on compliance efforts across the industry, any efficiency gained through automation and superior intelligence is extremely valuable. The future of pharmaceutical compliance involves a unified, real-time analytics platform that integrates global regulatory intelligence with internal operational data, ensuring that every product development, manufacturing, and commercial decision is made within a compliant framework.